CE certISO
As a company accredited by NAH, we certify quality management systems.
Our company is a certification body accredited by NAH, so we are authorised to perform certification according to standard ISO 13485:2016.
Information on the certification procedure:
Our certification procedure of MSZ EN ISO 13485:2016 is shown in the following illustration:
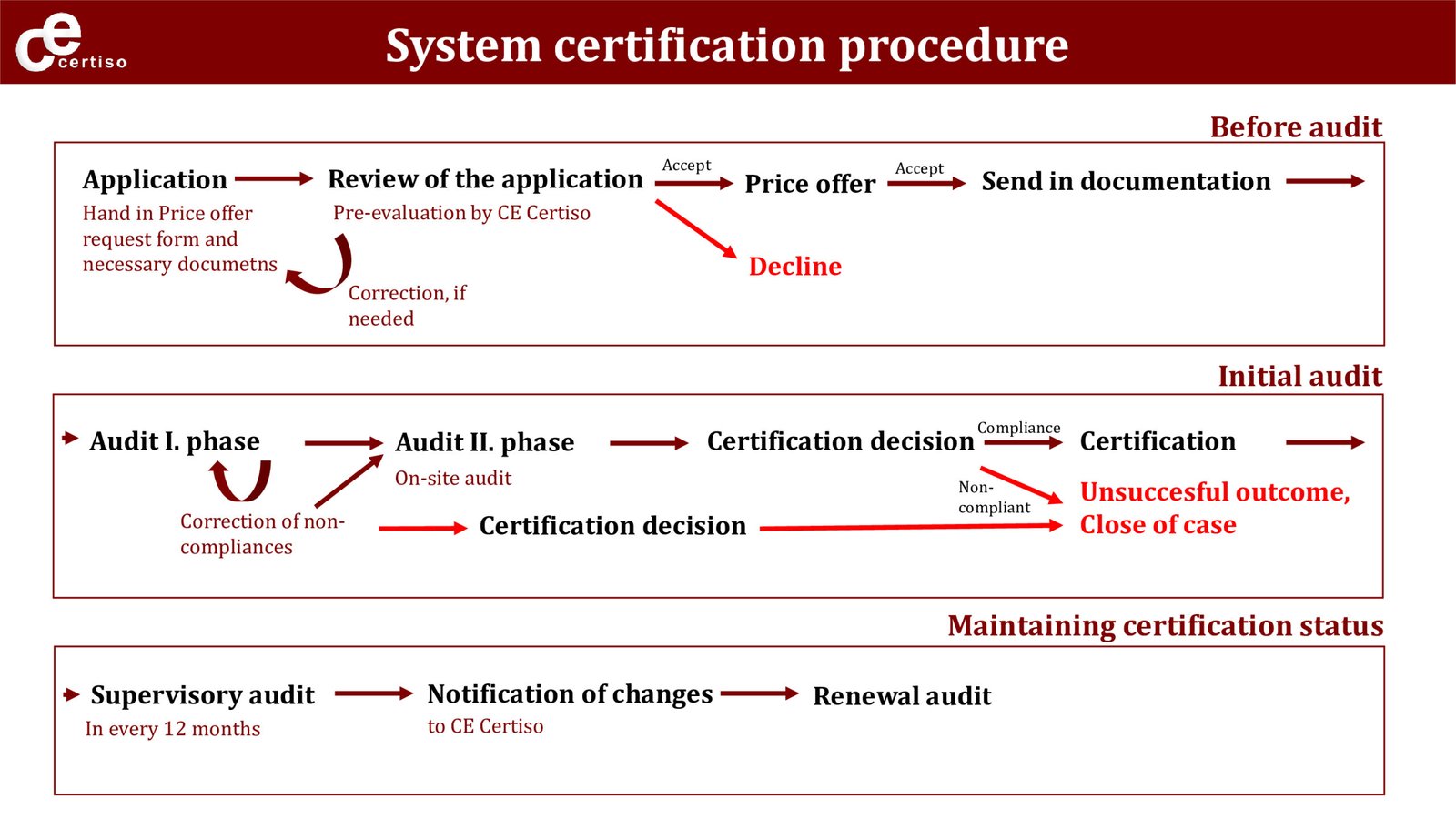
The System certification audit has two stages: Stage 1. and Stage 2.
STAGE 1: The certification procedure begins with the stage 1. We evaluate the quality management system documentation – we request our clients to submit
1) the quality manual and main procedures,
2) the list of valid quality management procedures,
3) the last internal audit report and
4) the last management review report.
We might ask for further quality management system documentation (procedures and in special cases work instructions etc.) as well. In case of Class III devices we shall finish the stage 1 audit with an onsite visit.
STAGE 2 (Stage 2 means the on-site audit and if applicable the verification of corrections and corrective actions and/or corrective action plan) can only be recommended by the audit team in case the reviewed quality management system documentation and/or other documentation allows it. “Allows” does not mean that there are not any nonconformities but there cannot be any nonconformity which would make the ability of the organization for the Stage 2 audit questionable. Notably, the documentation evaluated in the Stage 1 shall verify the preparedness of the company.
During the Stage 2 audit we can raise nonconformities against the quality management system and its operation.
During this period, we might ask for further corrections and/or documents (evidence).
Hereby we call your attention that in case CE Certiso Ltd is not able to verify the implementation of corrections and corrective actions of any major nonconformity within 6 months after the last day of stage 2 (on-site audit), we shall conduct another on-site audit prior to recommending certification.

Manufacture of medical devices is a special industrial branch since these devices and equipment are used in medicine, so the safety of them has enhanced importance in addition to the intended use.
Standard ISO 13485:2016 rules the procedures of manufacture of medical devices from the design to the application. It guarantees that the product fulfils the regulatory requirements of Hungary and the European Union.
Standard ISO 13485:2016 has been prepared for such organisations that, for using the CE mark on their products, shall conform one or more „New Approach” European directives on medical devices.
A certified ISO 13485 quality management system provides the following added values:
During the validity of the certificate, an external and independent certification organization proves that your procedures fulfil the requirements of the standard.
You’re entitled to use our certification mark
You can get a priority in public procurements
A list on withdrawn, suspended and forged certificates can be found under the Certificate information menu
The list of our certified clients is available upon request. Please submit your requests in writing to our addresses.
